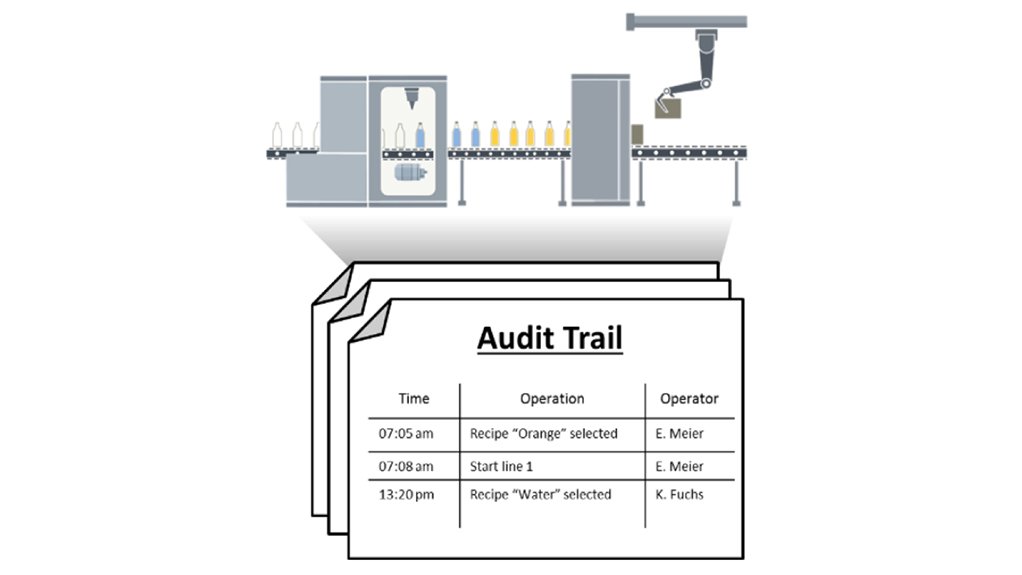
In industries, e.g. the pharmaceutical industry, or the food industry, there are various regulations regarding the traceability and documentation of production data. These include, among others, the following regulations. Complete tracing of operator control actions carried out by operators, using a timestamp (creation of audit-trails). Protection of important process steps using an electronic signature. Archiving of accountable user actions. The option package SIMATIC WinCC Audit (TIA Portal) supports you in implementing a GMP-compliant configuration. Via this option package, you can also automatically create, document and archive the Audit Trails. Below are some details of an example solution using the below layout components shown.
The solution presented here offers the following advantages:
1.Time and cost savings
2.Reducing the engineering effort
3.Electronic recording of Audit Trails as opposed to paper documentation
GMP stands for "Good Manufacturing Practice”. The term refers to certain guidelines designed to ensure the quality of production of, e.g. pharmaceuticals, substances, cosmetics and food. Next to production, the GMP guidelines also demand high quality standards on processing, packaging and storage. If the GMP guidelines are not complied with, companies are not allowed to manufacture the respective products. The most important regulatory basis is the Title 21 CFR Part 11 of the US Food and Drug Administration on electronic data recording and electronic signatures. Further country-specific and industry-specific regulations also apply. Note Before implementing a project, observe the respective country- and industry specific laws and regulations concerning GMP.
WinCC Audit (TIA Portal) is an option package of WinCC (TIA Portal). This gives you extended functions of WinCC (TIA Portal) Engineering, whereby you can ensure the GMP compliance of your project. If you activate the WinCC Audit (TIA Portal) option, the following additional functions will be made available:
1.Archive Audit Trail
2.Audit Trail Protocol for printing archived changes
3.System function "Notify User Action"
4."GMP relevant” markings for tags and recipes possible
5.Electronic signature
6.Archiving tags and messages with checksum
The WinCC Audit (TIA Portal) option is available for the following control panels:
-
2nd Generation Mobile panels
-
Comfort panels
-
WinCC Runtime Advanced
In order to be able to use the functions of WinCC Audit (TIA Portal) in the Runtime of the operator panel, you need to license each used HMI control panel individually. The following licenses are available for this purpose:
SIMATIC WinCC Audit for SIMATIC Panels: 6AV2107-0RP00-0BB0
SIMATIC WinCC Audit for Runtime Advanced: 6AV2107-0RA00-0BB0.
The user administration plays a central role for a GMP-compliant manufacturing process. In the pharmaceutical industry, for example, it must be ensured that recipe data can only be changed by authorized personnel. Depending on the plant concept, you can implement the following two types of user administration:
-
Local user administration
-
Central user (using SIMATIC Logon)
Siemens